Glucagon-like peptide-1 (GLP-1) is a GI peptide that stimulates insulin secretion (similar to sulfonylureas). GLP-1 also inhibits glucagon release, gastric emptying and food absorption. GLP-1 and another similar peptide are called incretins. As noted above, incretins have a dual action which leads to lowering blood glucose:
1. Stimulate insulin release
2. Inhibit glucagon release
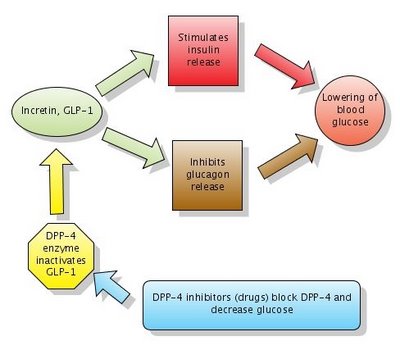
Figure 1. Action of DPP-4 inhibitors. Note that DPP-4 normally inactivates GLP-1. DPP-4 inhibitors block DPP-4 which in turn leaves GLP-1 active. Click to enlarge the figure. Created with Gliffy. The diagram Action of DPP-4 inhibitors is now on Wikipedia.
Exenatide (Byetta) is a GLP-1 receptor agonist approved for adjunctive therapy for patients with DM 2 who are not well controlled on oral agents. It is available only as injections and has to be administered twice daily.
Two new medications increase GLP-1 levels by blocking the enzyme which inactivates GLP-1. The enzymes is called DPP-4 (dipeptidyl peptidase-4) and the new medications are called DPP-4 inhibitors or gliptins.
They act similarly to Byetta (see figure above) but have the big advantage to be available in oral form (pills). These 2 new medications for treatment of DM2 are:
- Sitagliptin (Januvia) is taken once a day and it costs about $ 4.50 per pill
- Vildagliptin (Galvus) is waiting for FDA approval but it is already speculated to be at a competitive disadvantage to Januvia because it has to be taken twice a day
References:
New Medications
Merck Wins U.S. Approval for a New Diabetes Drug. NYTimes.
Sitagliptin: First DPP-4 Inhibitor for Type 2 Diabetes. Resident and Staff Physician, 04/2007.
Merck Diabetes Drug Could Face Competition from Takeda. WSJ Health Blog, 01/2008.
Related:
Lilly, Amylin Disclose More Cases of Byetta-Related Pancreatitis. WSJ Health Blog, 08/2008.
FDA Issues Warning for Diabetes Drug Byetta about possible kidney problems, including renal failure http://bit.ly/1UOjwB
Updated: 11/03/2009
1. Stimulate insulin release
2. Inhibit glucagon release

Figure 1. Action of DPP-4 inhibitors. Note that DPP-4 normally inactivates GLP-1. DPP-4 inhibitors block DPP-4 which in turn leaves GLP-1 active. Click to enlarge the figure. Created with Gliffy. The diagram Action of DPP-4 inhibitors is now on Wikipedia.
Exenatide (Byetta) is a GLP-1 receptor agonist approved for adjunctive therapy for patients with DM 2 who are not well controlled on oral agents. It is available only as injections and has to be administered twice daily.
Two new medications increase GLP-1 levels by blocking the enzyme which inactivates GLP-1. The enzymes is called DPP-4 (dipeptidyl peptidase-4) and the new medications are called DPP-4 inhibitors or gliptins.
They act similarly to Byetta (see figure above) but have the big advantage to be available in oral form (pills). These 2 new medications for treatment of DM2 are:
- Sitagliptin (Januvia) is taken once a day and it costs about $ 4.50 per pill
- Vildagliptin (Galvus) is waiting for FDA approval but it is already speculated to be at a competitive disadvantage to Januvia because it has to be taken twice a day
References:
New Medications
Merck Wins U.S. Approval for a New Diabetes Drug. NYTimes.
Sitagliptin: First DPP-4 Inhibitor for Type 2 Diabetes. Resident and Staff Physician, 04/2007.
Merck Diabetes Drug Could Face Competition from Takeda. WSJ Health Blog, 01/2008.
Related:
Lilly, Amylin Disclose More Cases of Byetta-Related Pancreatitis. WSJ Health Blog, 08/2008.
FDA Issues Warning for Diabetes Drug Byetta about possible kidney problems, including renal failure http://bit.ly/1UOjwB
Updated: 11/03/2009